
CHEARS News Room
This is News Broadcast Room of CHEARS.
Our purpose is to create awareness among general public through the various health news updates and interventional updates from the sector of healthcare. This News Room works under Dr. Keerthana Ramesh, Chief Editor, Department of CNAR, CHEAARS.
First-of-its-Kind Automated Insulin Delivery and Monitoring System for Use in Young Pediatric Patients
1 September 2020
U.S. Food and Drug Administration approved the MiniMed 770G System, a hybrid closed loop diabetes management device that is intended to automatically monitor glucose (sugar) and provide appropriate basal insulin doses with little or no input from the users or their caregivers for use by individuals aged 2 to 6 with type 1 diabetes. The 770G System is a first-of-a-kind device for patients aged 2 to 6 years. It is the first legally marketed device that can automatically adjust insulin delivery based on continuous glucose monitor values for this patient population.
“Advancements in science, technology and manufacturing have helped make great strides in the treatment and successful management of type 1 diabetes, a life-threatening chronic condition,” said FDA Commissioner Stephen M. Hahn, M.D. “The FDA is dedicated to promoting policies that support the development of new technologies based on these advances, and remains committed to helping ensure that development and expansion of products that can improve the quality of life for those with this condition—which can particularly impact children—is safe and effective.”
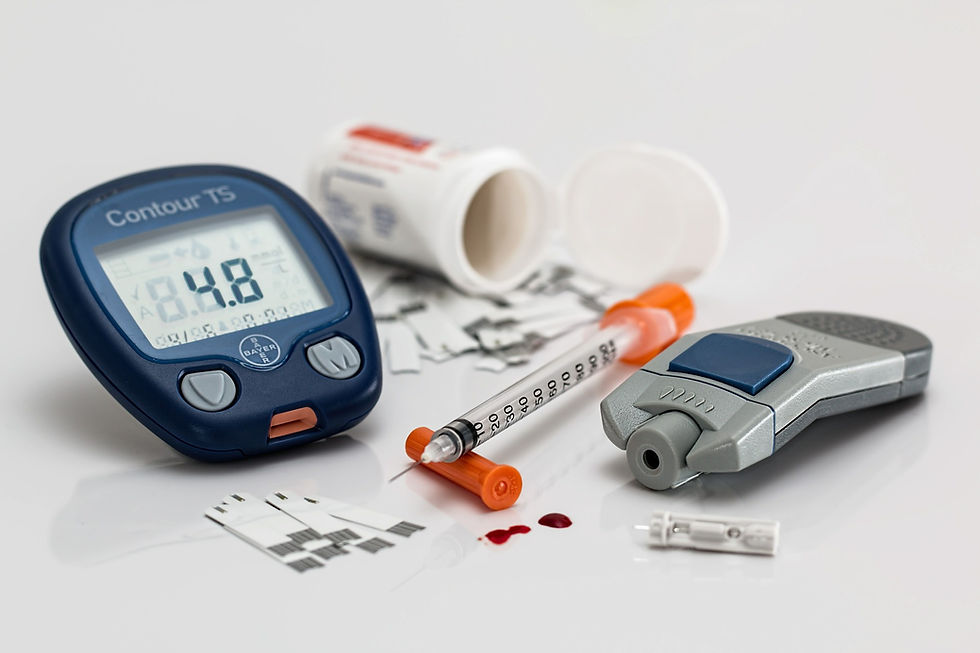
Patients with Type 1 diabetes, or their caregivers, must consistently monitor their glucose levels throughout the day and inject insulin with a syringe, pen or pump to maintain adequate glucose levels in order to avoid becoming hyperglycemic (high glucose levels) or hypoglycemic (low glucose levels).
The MiniMed 770G System, a bluetooth-enabled version of the previously approved MiniMed 670G System (with other modifications), is a hybrid closed loop system that works by measuring glucose levels in the body every five minutes and automatically adjusting insulin delivery by either administering or withholding insulin. The system includes: a sensor that attaches to the body to measure glucose levels under the skin; an insulin pump strapped to the body; and an infusion patch connected to the pump with a catheter that delivers insulin. While the device automatically adjusts insulin levels, users need to manually request insulin doses to counter carbohydrate consumption at mealtime.
The FDA evaluated data from a clinical trial that included 46 children aged 2 to 6 years old with type 1 diabetes. Study participants wore the device for approximately three months to evaluate the performance of the device during both the at-home periods, as well as a hotel period, to stress the system with sustained daily exercise. That study found no serious adverse events and that the device is safe for use. Data from that study was used to help support the expanded indication for patients 2 to 6 years old.
Risks associated with use of the system may include hypoglycemia, hyperglycemia, as well as skin irritation or redness around the device’s infusion patch. As part of this approval, the FDA is requiring the device manufacturer to conduct a post-market study to evaluate device performance in real-world settings in children between the ages of 2 and 6.
This device is not approved for use in children younger than 2 years old and in individuals who require less than eight units of insulin per day.
The approval of the MiniMed 770G hybrid closed loop system was granted to Medtronic.
17
It will be great, if you share your view on above write-up.
Your content has been submitted. Please refresh page to see comment
Dr. Keerthana Ramesh
12 September 2020 at 7:08:59 am
Really worth



